Which Best Describes the Origin of Crystal Field Splitting
Our model crystal field plasmon splitting is based on secular equations for geometric eigenmodes allowing for the separation of pure shape from materials. A very small difference in separation results in a large difference in.
What Do You Mean By A Crystal Field Stabilization Energy Quora
Give the labels of each set of orbitals.

. In this paper Bethe was one of the first to give point group symmetry arguments to solve a quantum mechanical problem. The removal of a pair of ligands from the z-axis of an octahedron leaves four ligands in the x-y plane. The splitting of a mineral along.
Chemical bonds created by sharing a pair of electron between atoms. In this paper Bethe was one of the first to give point group symmetry arguments to solve a quantum mechanical problem. Crystal field splitting number is denoted by the capital Greek letter Δ.
A substance whose atoms are arranged in a regular orderly periodically repeated pattern. Eg t2go 10Dq-25o -4Dq 35o 6Dq energy R3 Oh Energy level of hypothetical spherical field Crystal Field Splitting Energy ΔoThe energy gap between t2g and eg levels is designated Δo or 10DqThe energy increase of the eg orbitals and the energy decrease of the t2g orbitals must be balanced relative to the energy of the hypothetical spherical field sometimes called the. B c What factors determine whether an octahedral complex is high or low spin.
When the ligands approach the central metal ion the degeneracy of electronic orbital states usually d or f. The theory was founded in 1929 by Hans Bethe. Thus the splitting breaks the degeneracy of dx2-y2 and dz2.
The theory was founded in 1929 by Hans Bethe. Crystal Field Theory History Crystal field theory is a quantum mechanical theory for the explanation of magnetic properties and colors of transition metal complexes. The explanation is that the crystal field splitting is very sensitive to the distance of separation of the polymer chains.
A strong repulsion with the electron and ligand take place in between the platinum which leads to a strong crystal field splitting. Crystal Field Theory History Crystal field theory is a quantum mechanical theory for the explanation of magnetic properties and colors of transition metal complexes. The complex that has highest crystal field splitting energy triangle is.
It arises due to the fact that when the d-orbitals are split in a ligand field as described above some of them become lower in energy than before with respect to a spherical field known as the bari centre in which all five d. What powers the Earths external heat engine. They can approach the central metal ion either along the axis or in between the axis according to which splitting of d o.
3 a According to crystal field theory what is the origin of the splitting of the metal d- orbitals into two sets in an octahedral complex. When it is equal to 0 the complex is unstable. The crystal field stabilization energy CFSE is the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands.
Crystal Field Theory History Crystal field theory is a quantum mechanical theory for the explanation of magnetic properties and colors of transition metal complexes. Crystal field splitting explains the difference in color between two similar metal-ligand complexes. Crystal field splitting is the difference in energy between d orbitals of ligands.
Weak field Pe Strong field Pe Small High Spin Mostly d 8 Majority Low spin Strong field ligands ie Pd 2 Pt 2 Ir Au 3 d z2 d x2-y2 d xz d xy d yz d x2-y2 d z2 d xz d xy d yz d xz d z2 d x2-y2 d xy d yz 19. Which of the following describes a crystal. In which of the following subsystems is the Earths magnetic field generated.
Arrange following complex ions in increasing order of crystal field splitting energy Delta_0. Crystal field splitting is like octahedral splitting tetrahedral splitting or square planar splitting. In crystal field theory ligands are treated as point charges.
Crystal field splitting number is denoted by the capital Greek letter Δ. A According to crystal field theory what is the origin of the splitting of the metal d orbitals into two sets in an octahedral complex. B Show the splitting of the metal d-orbitals in an octahedral complex.
The intermolecular interaction forces fall off at a rate of r 6 where r is the distance between chains. What is crystal field splitting in chemistry. The removal of the two ligands stabilizes the d z2 level leaving the d x2-y 2 level as the most destabilized.
Asked Aug 24 2019 in Chemistry by Anup Agrawal 728k points class-12. Δ tends to increase with oxidation number and increases down a group on the periodic table. The crystal field stabilisation energy CFSE is the gain in the energy achieved by preferential filling up of orbitals by electrons.
We present an electromagnetic analogue of crystal or ligand field theory that describes geometric eigenmodes and the resonant plasmon wavelength in plasmonic nanocrystals in terms of a simple shape descriptor. Give the labels of each set of orbitals. Show the splitting of the metal d-orbitals in an octahedral complex.
C What factors determine whether an octahedral complex is high or low spin. It is usually less than or equal to 0. This degeneracy stabilizes more to the square planar arrangement than the tetrahedral.
The Crystal Field Theory CFT is a model for the bonding interaction between transition metals and ligands. It describes the effect of the attraction between the positive charge of the metal cation and negative charge on the non-bonding electrons of the ligand. Crystal field splitting explains the difference in color between two similar metal-ligand complexes.
Read PDF Crystal Field Theory History Crystal field theory - Simple English Wikipedia the free. The theory was founded in 1929 by Hans Bethe. ________ are groups of the same kinds of atoms that cannot be broken down into other substances by ordinary chemical means.
Therefore the crystal field splitting diagram for square planar geometry can be derived from the octahedral diagram. The problem is that very few polymers exhibit the crystal field splittings observed for PE. The _________ determines the chemical and physical properties of an atom.

Crystal Field Theory An Overview Sciencedirect Topics

Crystal Field An Overview Sciencedirect Topics

Crystal Field Theory An Overview Sciencedirect Topics

Crystal Field Theory An Overview Sciencedirect Topics
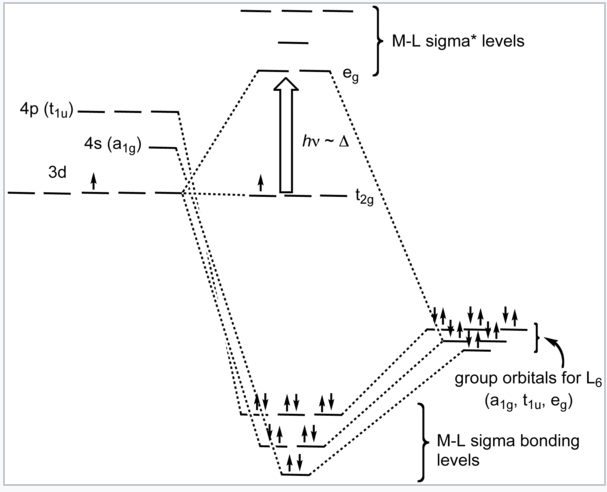
5 3 Crystal Field Theory Chemistry Libretexts

Orgonites The Complete Guide Orgonite Orgonite Pyramids Orgone Energy

Crystal Field Theory Introduction To Chemistry

Crystal Field An Overview Sciencedirect Topics

Crystal Field Theory An Overview Sciencedirect Topics
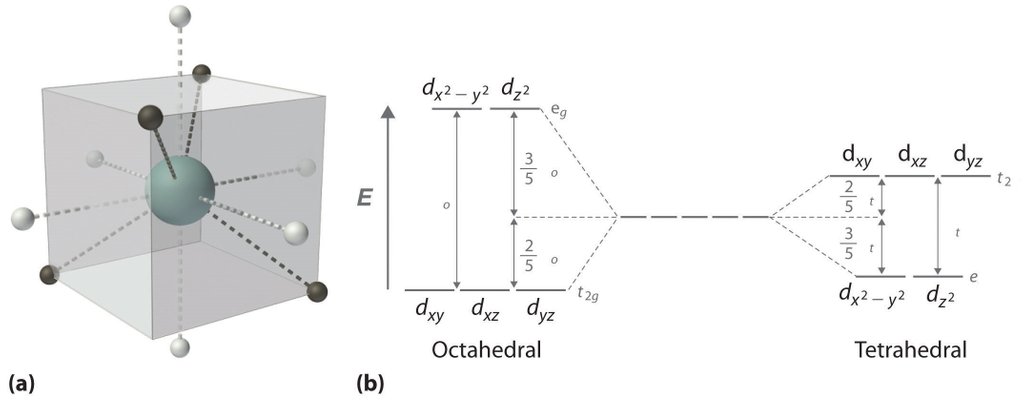
Crystal Field Theory Definition Examples Diagrams

Crystal Field Theory Introduction To Chemistry
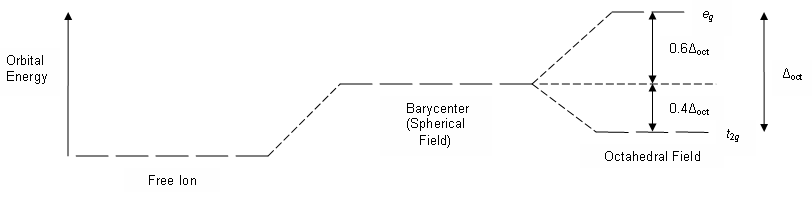
What Is Crystal Field Theory Quora

Spectroscopic And Magnetic Properties Of Coordination Compounds Chemistry For Majors
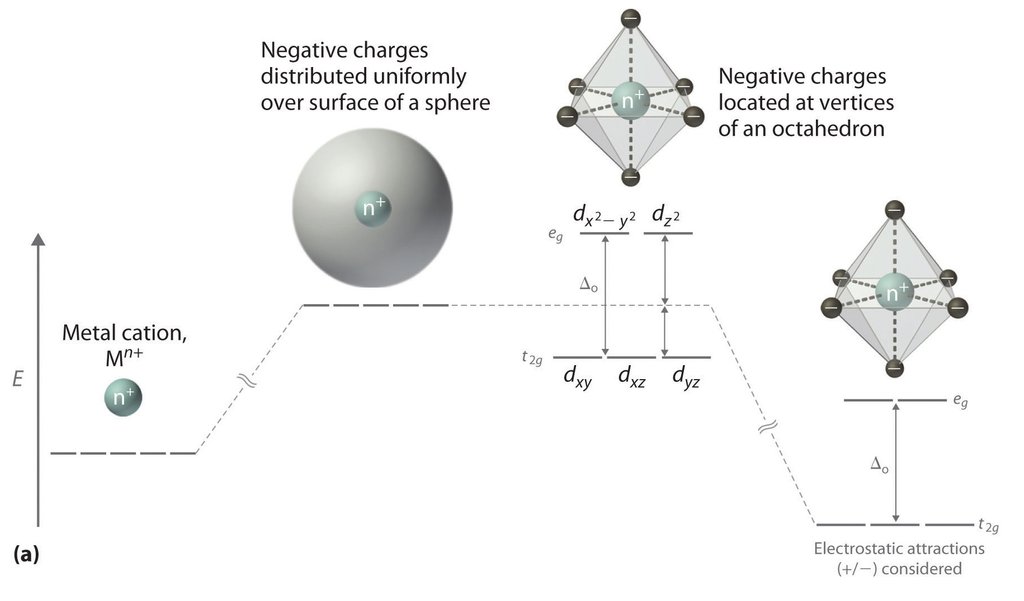
Crystal Field Theory Definition Examples Diagrams

What Is Crystal Field Splitting Energy How Does The Magnitude Of D0 Decide The Actual Configuration Of D Orbitals In A Coordination Entity


Comments
Post a Comment